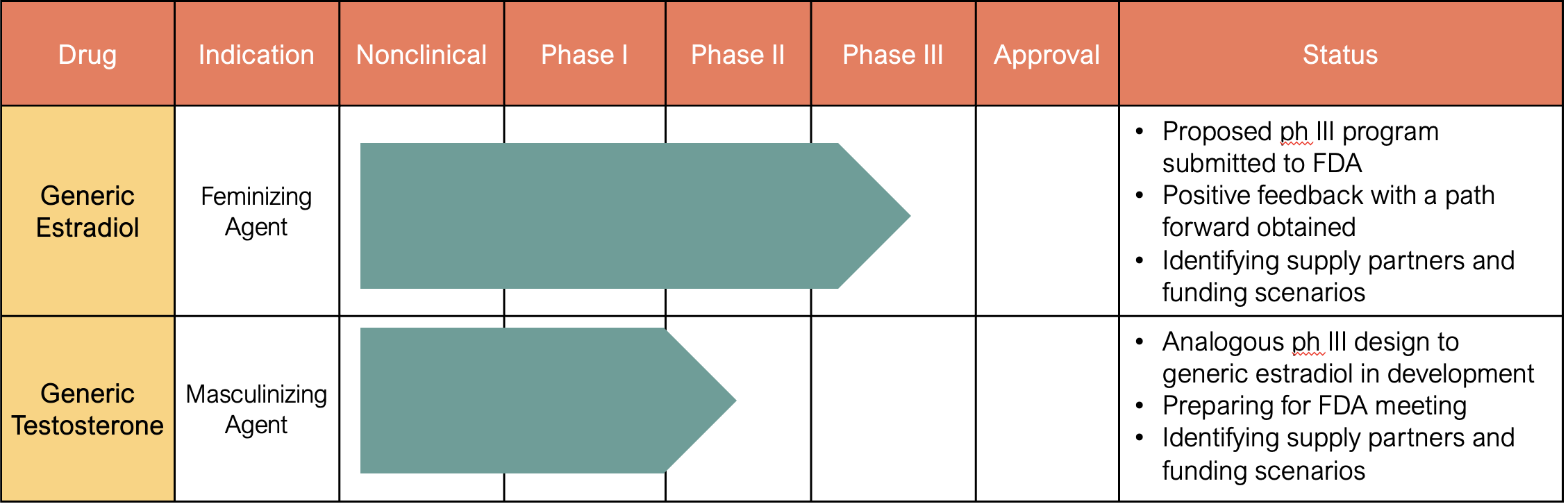
RIGT Pipeline & FDA Process
FDA approval refers to the process by which the U.S. Food and Drug Administration evaluates and grants authorization for pharmaceuticals, medical devices, and certain food products to be sold and marketed in the United States. This rigorous evaluation ensures that these products are safe, effective, and meet specific quality standards before they can be made available to the public.
FDA approval can offer protection against state regulations via FDA preemption. FDA preemption is legal concept that says the authority of the FDA to regulate and approve medical products takes precedence over state laws and regulations. When a drug is approved by the FDA, it may be shielded from certain legal actions or liability claims that could arise under state law.
A pipeline, in the context of pharmaceuticals and biotechnology, refers to the various stages of development that a potential new medication or therapy goes through before being FDA approved.
Phases
Part of the pipeline and FDA approval process are clinical trials. Clinical trials typically consist of several phases designed to evaluate the safety and efficacy of a new medical intervention, such as a drug or medical device. These phases are:
-
Phase I (Safety)
In this phase, a small group of healthy volunteers or individuals with the condition under study receives the experimental treatment. The primary goal is to determine the treatment's safety, dosage, and side effects. Researchers carefully monitor participants to establish the maximum tolerated dose.
-
Phase II (Efficacy and Side Effects)
Phase II involves a larger group of participants with the target condition. Researchers assess the treatment's effectiveness in treating the condition and continue to monitor safety and side effects. This phase provides valuable data to refine dosages and identify potential benefits.
-
Phase III (Confirmation)
Phase III trials involve a much larger and more diverse group of participants, often across multiple locations. The focus is on confirming the treatment's efficacy, monitoring side effects, and comparing it to existing standard treatments or placebos. The data generated in this phase is critical for regulatory approval.
-
Phase IV (Post-Market Surveillance)
After regulatory approval, Phase IV trials continue to monitor the treatment's long-term safety and effectiveness in a real-world setting. These trials help identify rare or long-term side effects and further refine usage guidelines.
“FDA approval for my HRT would provide reassurance that the drug has been tested for the purpose of treating gender incongruence and potentially allow for improved, US-based manufacturing, potentially reducing costs and eliminating manufacturing shortages that currently interrupt drug availability and pricing fluctuations, which are barriers to access." – Jamison Green, legacy advisor